The Anatomy of a Dry Cell: Types and Working Principle
2024/6/29 16:41:35
Views:
Everyday electronics, such as remote controls and torches, are powered by dry cells. Understanding their structure, types, and working principles unveils the science behind their widespread use and reliability.
Dry Cell Battery
Definition and Overview of Dry Cells

Dry Cell
A dry cell is an electrical power generator that relies on chemical reactions. When the two electrodes are connected in a closed circuit, the cell forces electrons to move from one end to the other through a fluid medium. This electron flow enables the generation of electricity within the closed system.
Dry cell batteries are among the simplest ways to produce electricity. Multiple cells combined together form a battery. The modern versions of dry cells include lead-acid or nickel-cadmium batteries. The dry cell was invented by French engineer Georges Leclanche in 1866, and his invention, known as Leclanche's battery, was initially quite heavy and prone to damage.
Today, dry cells come in various voltages and sizes, based on the same principle but as an improved variant of Leclanche's original design. In 1881, Carl Gassner from Mainz invented the commercial zinc-carbon cell, a modified version of the Leclanche battery. These cells are now manufactured in large quantities and are used in many applications, such as toys, radios, and calculators.
Chemical reactions allow the flow of electrons from one end to the other. When two or more cells are connected with correct polarity, a higher potential is created, causing more electrons to circulate. This assembly of cells is known as a battery, capable of achieving a wide range of voltages from as low as 1.5 V to as high as 100 V. Additionally, the DC output voltage of a battery can be controlled to different levels using power converters like chopper circuits.
Structure of Dry Cells
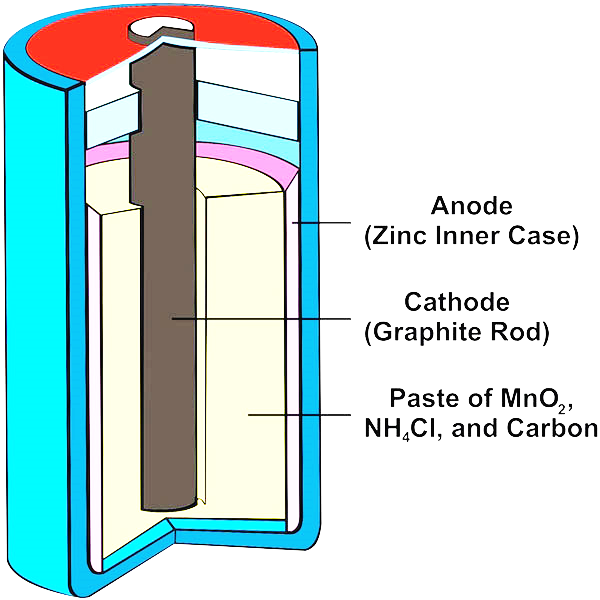
Dry cells are compact, portable electrochemical devices designed for single-use or intermittent use applications. Their design ensures efficient operation while being convenient for everyday use. Below, we will delve into the detailed structure and the role of each component in a typical dry cell.
1. Outer Casing
The outer casing of a dry cell is typically made of zinc. This casing serves a dual purpose:
- Negative Electrode (Anode): As the anode, the zinc casing undergoes oxidation, releasing electrons during the electrochemical reaction that powers the cell.
- Container: The zinc casing also acts as the physical container for the cell, housing all the other components securely. The choice of zinc is due to its effective electrochemical properties and its ability to form a sturdy, corrosion-resistant container.
Material Properties: Zinc is chosen for its excellent electrochemical properties and its ability to serve as both a container and an anode. Zinc's reactivity is harnessed in the cell's operation, and its corrosion resistance helps maintain the cell's integrity over time.
Manufacturing Process: The zinc casing is usually manufactured through a stamping or extrusion process, ensuring a robust and leak-proof container.
2. Electrolyte Paste
What sets a dry cell apart from other kinds of cells is that its electrolyte is a moist paste instead of a liquid. The paste typically consists of ammonium chloride (NH₄Cl) and zinc chloride (ZnCl₂). The functions of this electrolyte paste are as follows:
- Ion Flow Promotion: The electrolyte facilitates the movement of ions between the anode and cathode, which is essential for the electrochemical reactions to occur.
- Moisture Content: The moist nature of the paste allows for sufficient ionic conductivity while preventing leakage and allowing the cell to be used in various orientations.
Composition: The electrolyte paste is carefully formulated to ensure optimal ionic conductivity. Ammonium chloride and zinc chloride are chosen for their ability to provide a stable ionic environment.
Functionality: The moist nature of the paste is critical. It provides the necessary medium for ion exchange while preventing the cell from drying out, which would impede its performance.
3. Carbon Rod
At the core of the dry cell is the carbon rod, which serves as the positive electrode (cathode). A combination of graphite and manganese dioxide (MnO3) envelops the carbon rod. Each component has a specific function:
- Manganese Dioxide (MnO₂): This serves as the primary cathode material and is responsible for the reduction reactions during the cell's operation. MnO₂ helps in accepting electrons and reduces the formation of hydrogen gas, thereby reducing polarization and maintaining the efficiency of the cell.
- Graphite: Mixed with MnO₂, graphite serves as a conductor, improving the overall electrical conductivity of the cathode material.
Cathode Design: The carbon rod is central to the cell's operation. Its high conductivity and ability to support reduction reactions make it ideal for this role.
MnO₂ and Graphite Mix: The combination of manganese dioxide and graphite ensures that the cathode can efficiently accept electrons and maintain conductivity, minimizing internal resistance.
4. Separator
The separator in a dry cell is a crucial component made of a porous material, such as paper or synthetic fibers. Its primary functions include:
- Preventing Short Circuits: The separator ensures that the anode and cathode do not come into direct contact, which would cause a short circuit and render the cell useless.
- Allowing Ion Movement: While preventing direct contact, the separator is porous enough to allow ions to move freely between the electrodes, facilitating the electrochemical reactions necessary for the cell's operation.
Material Selection: The separator must be carefully chosen to balance porosity and mechanical strength. Materials like paper or synthetic fibers are selected for their ability to allow ion flow while preventing direct contact between electrodes.
Function in Electrochemical Reactions: By allowing ions to move while keeping the electrodes separate, the separator ensures that the electrochemical reactions can proceed efficiently without the risk of short-circuiting.
5. Terminal Cap
The terminal cap of a dry cell is usually made of brass or nickel-plated steel and is connected to the carbon rod. Its main roles are:
- Positive Terminal: The terminal cap acts as the positive terminal of the cell, providing a connection point for the external circuit.
- Conductivity: The materials used for the terminal cap, such as brass or nickel-plated steel, ensure good electrical conductivity and corrosion resistance, enhancing the cell's reliability and lifespan.
Connection Point: The terminal cap provides a reliable and conductive point for connecting the cell to external devices.
Material Durability: Brass and nickel-plated steel are chosen for their excellent conductivity and resistance to corrosion, ensuring that the terminal remains functional over the cell's lifespan.
Types of Dry Cells

Dry cells come in various types, each suited for specific applications and performance requirements. These are some of the most common types:
1. Carbon-Zinc Cells
Composition: Manganese dioxide serves as the cathode, zinc serves as the anode, and ammonium chloride paste serves as the electrolyte.
Characteristics: These are the most basic and inexpensive dry cells. They provide a nominal voltage of 1.5V and are used in low-drain devices.
Applications: Ideal for devices like flashlights, remote controls, and clocks.
2. Alkaline Cells
Composition: Potassium hydroxide (KOH) is used as the electrolyte in cells that resemble zinc-carbon cells.
Features: Alkaline batteries function better in high-drain situations and have a longer shelf life. Additionally, they offer a nominal voltage of 1.5V.
Uses: Found in high-drain gadgets like as toys, digital cameras, and portable electronics.
3. Zinc-Air Cells
Composition: Air (oxygen) as the cathode, potassium hydroxide as the electrolyte, and zinc as the anode.
Features: When exposed to oxygen, these cells activate, giving them a long shelf life and high energy density.
Applications: Commonly used in hearing aids, medical devices, and some small electronic devices.
4. Lithium Cells
Composition: Uses a non-aqueous electrolyte, a range of cathode materials (such carbon monofluoride or manganese dioxide), and lithium as the anode.
Features: The capacity to function in a broad temperature range, extended shelf life, and high energy density are their notable attributes. They provide a higher nominal voltage, typically around 3V.
Applications: Used in high-performance applications like cameras, watches, and medical implants.
5. Silver-Oxide Cells
Composition: An electrolyte of potassium hydroxide, silver oxide serving as the cathode, and zinc serving as the anode.
Characteristics: Offer a stable voltage and high energy density, with a nominal voltage of 1.55V.
Applications: Often used in small devices requiring stable voltage, such as watches, calculators, and some medical devices.
Working Principle of Dry Cells
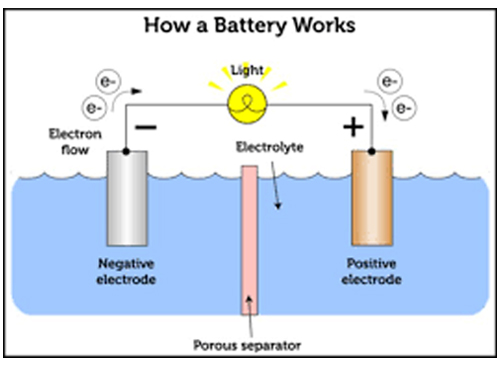
Dry cells generate electrical energy through redox reactions occurring between the anode and cathode. Here's a detailed look at the electrochemical processes:
1. Anode Reaction: At the anode, zinc undergoes oxidation, losing electrons:
Zn→ Zn2++ 2e-
2. Cathode Reaction: At the cathode, manganese dioxide (in zinc-carbon and alkaline cells) undergoes a reduction reaction. The exact reaction can vary, but typically:
In alkaline cells:
2MnO2+H2O+2e-→Mn2O3+2OH-
3. Electron Flow: Electrons released during the oxidation of zinc travel through the external circuit, providing power to the connected device. They then return to the cathode to complete the circuit.
4. Ion Flow: Inside the cell, ions move through the electrolyte. The movement of these ions helps maintain charge balance as the electrochemical reactions proceed.
5. Overall Reaction: The overall cell reaction combines the anode and cathode reactions:
Zn+2MnO2+2NH4Cl→ ZnCl2+Mn2O3+2NH3+H2O
Zn+2MnO2+2H2O+2H2O→Zn(OH)2+Mn2O3
Performance Characteristics of Dry Cells
The performance of dry cells is evaluated based on several key parameters, each of which determines the cell's suitability for various applications. These parameters include voltage, capacity, shelf life, discharge characteristics, energy density, and temperature performance. Choosing the best dry cell for a given set of requirements requires a full grasp of these features.
Voltage
Dry cells are known for their nominal voltage, which is typically 1.5V for most types, such as zinc-carbon and alkaline cells. Many different types of portable and home electronics can operate at this voltage. But because lithium batteries have a higher nominal voltage-roughly 3V-they can be used in high-performance applications that demand greater power.The stability of the voltage output is also an important factor. Alkaline cells are known for maintaining a stable voltage throughout most of their discharge cycle, whereas zinc-carbon cells experience a gradual voltage drop, which can affect the performance of devices that require steady voltage. Lithium cells excel in this area, providing very stable voltage throughout their discharge cycle, ideal for sensitive electronics.
Capacity
Capacity, measured in milliampere-hours (mAh), indicates the amount of charge a cell can deliver over time. Higher capacity means the cell can power a device longer before needing replacement. Zinc-carbon cells generally have lower capacity, suitable for low-drain applications. Alkaline cells offer higher capacity, providing longer operation times for moderate to high-drain devices. Lithium cells boast the highest capacity, supporting prolonged use in high-drain and high-energy applications.Shelf Life
The amount of time a cell may be kept without experiencing a noticeable decrease of capacity is called its shelf life. Zinc-carbon cells have a shorter shelf life, typically around 1-3 years, due to the gradual depletion of the anode material and leakage issues. Alkaline cells provide a longer shelf life, typically 5-10 years, thanks to better materials and construction. Lithium cells exhibit the longest shelf life, often exceeding 10 years, with minimal self-discharge and high stability over time.Discharge Characteristics
Discharge characteristics describe how the voltage of the cell changes over time as it discharges. Zinc-carbon cells experience a significant and continuous voltage drop, which can lead to reduced performance in devices requiring consistent power. Alkaline cells maintain a more stable voltage profile, with a gradual decline only towards the end of their life, making them preferable for many electronic devices. Lithium cells offer the most stable voltage during discharge, ensuring consistent performance in high-drain devices and critical applications.Energy Density
The quantity of energy a cell can hold in relation to its volume or weight is known as its energy density. Zinc-carbon cells have the lowest energy density, limiting their use to low-energy applications. Alkaline cells provide higher energy density, suitable for more demanding applications. Lithium cells possess the highest energy density, making them ideal for compact, high-energy applications where space and weight are crucial, such as in medical devices and advanced electronics.Temperature Performance
Temperature performance refers to the range of temperatures over which a cell can effectively operate. Zinc-carbon cells perform poorly at low temperatures, with significantly reduced capacity and efficiency. Alkaline cells have a wider operating temperature range, performing well in both low and moderately high temperatures, making them more versatile. Lithium cells exhibit the best temperature performance, functioning effectively in temperatures from as low as -40°C to as high as 60°C, suitable for extreme conditions such as outdoor sensors and aerospace applications.
The performance parameters of dry cells must be assessed in order to determine which kind is best for a certain application. Zinc-carbon cells are suitable for low-drain, cost-sensitive applications where high performance is not critical. Alkaline cells are ideal for a broad range of devices requiring moderate to high power, with good stability and shelf life. Lithium cells are best for high-drain, high-energy applications needing long shelf life and excellent performance across a wide temperature range.
Understanding these performance metrics ensures the optimal use of dry cells, enhancing device efficiency, reliability, and longevity. By considering these parameters, consumers and engineers can make informed decisions, selecting the most appropriate dry cell type to meet their specific requirements.
Dry batteries vs Wet batteries
Dry Cell:
- A paste electrolyte is used in dry cell batteries, reducing the possibility of leakage.- It is commonly portable and found in commonplace gadgets like clocks, remote controls, and flashlights.
- A carbon rod cathode and a zinc anode are used in the construction, and they are encircled by manganese dioxide paste.
- Since the electrolyte is in paste form, it allows the cell to be operated in any orientation without spillage.
- Examples of dry cells include the popular alkaline batteries and zinc-carbon batteries.
Wet Cell:
- wet cell, also known as a liquid cell, uses a liquid electrolyte, which can be an acid or a base.- Since these cells are frequently non-portable, handling them carefully is necessary to avoid leaks.
- Wet cells are often used in automotive applications, such as car batteries, and in stationary applications like backup power for telecommunications.
- Lead and lead dioxide plates dissolved in a sulfuric acid solution are commonly used in construction.
- Wet cells need to be kept upright and are usually encased in a robust container to handle the corrosive liquid electrolyte.
Based on their features and designs, moist and dry cells have distinct uses. Dry cells are ideal for portable, low to moderate power applications with their sealed, maintenance-free design. Wet cells, on the other hand, are suited for high-power, heavy-duty applications but require regular maintenance and careful handling due to their liquid electrolyte and potential for spillage. It is essential to comprehend these variations in order to choose the appropriate kind of cell for a given application.
Pros and Cons of Dry Cells
Pros of Dry Cells:
- Portability: Dry cells are small, lightweight, and easy to transport, making them ideal for portable devices.
- Leak-Proof: The paste electrolyte minimizes the risk of leakage, allowing dry cells to be used safely in any orientation.
- User-Friendly: Widely available and easy to replace, dry cells are convenient for everyday use in household gadgets.
- Low Maintenance: Unlike some batteries that require regular maintenance, dry cells generally need no upkeep.
- Affordable: Dry cells are relatively inexpensive, providing a cost-effective solution for small-scale applications.
- Versatile Sizes: They support a broad range of devices, from flashlights to remote controls, and come in a variety of sizes and capacities.
Cons of Dry Cells:
- Limited Lifespan: They have a finite lifespan and may require frequent replacement, especially in high-drain devices.- Lower Energy Density: Dry cells store less energy for their size compared to some other battery types, like lithium-ion batteries.
- Environmental Concerns: Disposal can pose environmental hazards due to the presence of chemicals and heavy metals, necessitating proper recycling.
- Cold Weather Performance: Their efficiency and operating time can decrease significantly in very cold temperatures.
- Corrosion Risk: Over time, internal chemical reactions can cause corrosion, potentially damaging the devices they power if leakage occurs.
Environmental Impact and Disposal of Dry Cells

Dry cells, while essential for powering numerous devices, pose significant environmental concerns if not disposed of properly. The materials used in these cells, particularly in zinc-carbon and alkaline types, can be harmful to the environment. Minimizing these dangers requires an understanding of the environmental impact and appropriate disposal techniques.
Recycling
Recycling dry cells is an effective way to mitigate their environmental impact. Many components of dry cells, especially metals, can be recovered and reused. The recycling process involves several steps:
1. Collection: Used batteries are collected from consumers, often through designated drop-off points at retailers, recycling centers, or community collection events.
2. Sorting: Batteries are sorted by type and chemistry to ensure they are processed correctly.
3. Processing: After being processed, the batteries are taken to recycling centers and disassembled. Different mechanical and chemical techniques are used to extract metals including steel, zinc, and manganese.
4. Reuse: By using the recovered materials to make new batteries or other products, less raw material needs to be mined and processed, protecting natural resources.
Recycling saves resources by reintroducing important metals into the production cycle, which also lessens pollution to the environment. However, consumer knowledge and involvement are key components of recycling programs' efficacy.
Toxicity
If not handled appropriately, dry cells may contain hazardous materials that endanger human health and the environment. Some of the key toxic elements include:
1. Heavy Metals: Older batteries, particularly those manufactured before regulations restricted their use, can contain heavy metals such as mercury, cadmium, and lead. These metals are extremely hazardous, and if they seep into soil and water systems, they have the potential to seriously harm the ecosystem.
2. Electrolytes: The chemicals used in the electrolyte paste, such as ammonium chloride and potassium hydroxide, can also be harmful. They can cause soil and water contamination, affecting plant and animal life.
Proper disposal methods are essential to mitigate these hazards. Consumers are encouraged to:
- Return Used Batteries: Many retailers and manufacturers offer take-back programs where consumers can return used batteries for proper disposal and recycling.
- Use Designated Recycling Centers: Community recycling centers often accept used batteries and ensure they are processed in an environmentally safe manner.
- Avoid Landfill Disposal: Disposing of batteries in regular trash can lead to toxic substances leaching into the environment. It is crucial to use designated battery disposal and recycling options.
Regulations
To address the environmental impact of dry cells, many countries have implemented regulations governing their disposal and recycling. These regulations aim to minimize environmental harm and promote sustainable practices. Key aspects of these regulations include:
1. Manufacturer Responsibility: In some regions, manufacturers are required to take back used batteries and ensure they are recycled properly. With extended producer responsibility (EPR), producers bear the weight of disposal instead of consumers.
2. Targets for Battery Collection and Recycling: Specific goals are frequently established by regulations for battery collection and recycling. As an illustration, member states are required by the European Union's Battery Directive to attain a minimum rate of collection for portable batteries.
3. Hazardous Substance Restrictions: Many countries have laws that restrict or ban the use of certain hazardous substances in batteries. For instance, the EU's Restriction of Hazardous Substances (RoHS) directive limits the use of mercury, cadmium, and other toxic materials.
4. Consumer Education: To inform the public about the value of properly disposing of batteries and the range of recycling choices, governments and organizations launch awareness campaigns.
Environmental Best Practices
To further reduce the environmental impact of dry cells, consumers and manufacturers can adopt several best practices:
1. Use Rechargeable Batteries: Where possible, use rechargeable batteries instead of disposable ones. Because they may be reused numerous times, rechargeable batteries cut down on the quantity of batteries that need to be disposed of.
2. Choose Eco-friendly Brands: Some manufacturers produce batteries with a reduced environmental impact, such as those with lower levels of toxic substances or higher recyclability.
3. Appropriate Handling and Storage: To avoid leaks and damage, keep batteries in a cool, dry location. Batteries can leak and have a shorter lifespan if fresh and old batteries are combined in electronics.
4. Participate in Recycling Programs: Actively participate in available recycling programs and encourage others to do the same.
The environmental impact of dry cells, particularly zinc-carbon and alkaline types, can be significant if not managed properly. Reducing these effects requires recycling, appropriate disposal, and compliance with laws. By understanding the toxicity of battery components and participating in responsible disposal practices, consumers can help reduce environmental pollution and conserve resources. Rules are essential in ensuring that both consumers and manufacturers contribute to the sustainable use and disposal of batteries. Adopting best practices, such as using rechargeable batteries and supporting eco-friendly brands, further enhances these efforts, promoting a healthier environment for future generations.
Common Queries Answered
1. What is the difference between a dry cell and a rechargeable battery?
The dry cell is one type of primary battery that is meant to be used once and cannot be recharged. Because of the paste electrolyte it utilizes, leakage is reduced. On the other hand, a secondary battery that is rechargeable has the ability to be used repeatedly. Different chemical compositions, such as lithium-ion, nickel-cadmium, or lead-acid, are commonly used in rechargeable batteries.
2. How does a dry cell work?
The components of a dry cell react chemically to produce electricity. It is made up of a carbon cathode and a zinc anode separated by an electrolyte paste. Chemical reactions take place when the circuit is closed, allowing electrons to move from the anode to the cathode via an external circuit and producing an electric current.
3. How long does a dry cell last?
The lifespan of a dry cell depends on several factors, including its size, the amount of current it supplies, and the conditions of its usage. Typically, dry cells can last anywhere from a few months to several years in storage. Under continuous use, they might last for a few hours to a few days, depending on the device they power.
4. How should dry cells be disposed of and recycled properly?
Dry cells should be disposed of in accordance with local laws to reduce their influence on the environment. Many communities offer battery recycling programs or hazardous waste collection events. It's important to keep used batteries out of regular trash to prevent soil and water contamination from heavy metals.
5. What are the main applications of dry cells?
Dry cells are widely used in everyday devices, including: Flashlights, Remote controls, Toys, Clocks, Portable radios, Calculators, Smoke detectors.
6. What is the history and who invented the dry cell?
The dry cell was invented by French engineer Georges Leclanche in 1866. His invention, known as Leclanche's battery, was initially quite heavy and prone to damage. The commercial zinc-carbon dry cell, an improved version of Leclanche's design, was developed by Carl Gassner in 1881.
7. What is the difference between a dry cell and a wet cell?
The primary difference lies in the electrolyte. Because a paste or gel electrolyte is used in dry cells, they are less likely to leak and are more portable. A wet cell, on the other hand, uses a liquid electrolyte, which can spill and is typically used in stationary applications like car batteries.
8. How do you choose the voltage and capacity of a dry cell?
The voltage and capacity of a dry cell depend on the requirements of the device it will power. Standard dry cells, like AA, AAA, C, and D batteries, typically have a voltage of 1.5V. The battery's milliampere-hour (mAh) capacity tells us how long it can run a given current. Devices that use batteries for longer periods of time or consume more power are better suited for larger capacity batteries.
9. How do you test the charge of a dry cell?
You can test the charge of a dry cell using a multimeter. Connect the positive lead to the positive terminal of the battery and the negative lead to the negative terminal once the multimeter has been set to detect DC voltage. A fully charged 1.5V dry cell should read around 1.5V to 1.6V. It could be necessary to replace the battery if the voltage drops noticeably.
10. Can dry cells leak, and what should you do if they do?
Yes, dry cells can leak, especially if they are old, damaged, or improperly stored. Leaking batteries should be handled with care. Wear gloves, clean the affected area with a mild solution of vinegar or lemon juice for alkaline batteries, or baking soda for acidic batteries, and dispose of the batteries according to local regulations. Avoid contact with skin and eyes.
Related Information
-
-
Phone
+86 135 3401 3447 -
Whatsapp





