Lithium-Ion Battery Basics: Understanding Structure and Working Principles
2024/6/25 10:48:45
Views:
Electric automobiles and cellphones alike are powered by lithium-ion batteries, which are now widely employed in modern technology. Their extended cycle life, minimal self-discharge, and high energy density are the main reasons for their appeal. We shall examine the composition, operation, and packaging of lithium-ion batteries in this extensive blog post.
How do Lithium-ion Batteries Work?
- Ⅰ. Introduction
- Ⅱ. Structure of Lithium-ion Batteries
- Ⅲ. Working Principle of Lithium-ion Batteries
- Ⅳ. Packaging of Lithium-ion Batteries
- Ⅴ. Primary apparatus for producing lithium-ion batteries
- Ⅵ. Advantages and Challenges of Lithium-ion Batteries
- Ⅶ. Future Developments in Lithium-ion Batteries
- Ⅷ. Conclusion
-
Ⅰ. Introduction

Figure 1
In a lithium-ion battery, which is a rechargeable energy storage and release device, lithium ions move between the anode and cathode via an electrolyte. Graphite is frequently utilized as the anode and lithium metal oxides, including cobalt oxide or lithium iron phosphate, as the cathode. When charging or discharging, lithium ions move electrical power from the cathode to the anode and back again. These batteries are preferred because of their low self-discharge rate, extended cycle life, and high energy density, which make them perfect for usage in electric cars, portable gadgets, and renewable energy storage systems.
Ⅱ. Structure of Lithium-ion Batteries
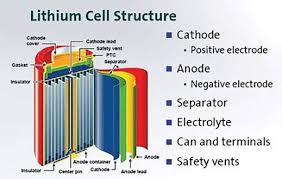
Figure 2
Lithium-ion batteries are sophisticated energy storage devices with several key components working together to provide efficient and reliable power. Understanding each component's role and characteristics is essential for appreciating the battery's overall functionality. Here, we will delve deeper into the structure of lithium-ion batteries, covering each major component in detail.
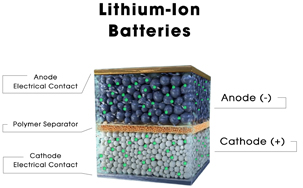
Figure 3
1. Anode
An essential part of a lithium-ion battery is the anode, which is usually composed of graphite. Graphite is favored due to its unique properties, which include:
- ● Layered Structure: Graphite's layered structure allows lithium ions to intercalate (insert) between the layers easily. This intercalation process is reversible, enabling repeated charging and discharging cycles.
- ● High Electrical Conductivity: Graphite conducts electricity well, facilitating efficient electron flow during battery operation.
- ● Consistency: Graphite is resistant to considerable degradation even after many cycles of charge and discharge due to its chemical stability.
During the charging process, lithium ions move from the cathode to the anode and are held in the spaces between the graphite layers. The battery's high energy density is a result of this effective storing method.
Different Materials for Anodes:
- ● Silicon: Silicon can store more lithium ions than graphite because of its significantly larger theoretical capacity. However, silicon expands significantly when it absorbs lithium, which can cause mechanical stress and degradation over repeated cycles. Researchers are looking on ways to lessen this problem, including composite materials or silicon nanostructures.
- ● Lithium Titanium Oxide (LTO): LTO offers excellent safety and fast charging capabilities, as it does not form a solid-electrolyte interphase (SEI) layer and has a higher lithium diffusion rate. In contrast to graphite, it has a lower energy density.
2. Cathode
Another essential part of a lithium-ion battery that is formed of lithium metal oxides is the cathode. The capacity, functionality, and safety of the battery are significantly impacted by the cathode material selection. Typical cathode components consist of:
- ● Lithium Cobalt Oxide (LiCoO2): LiCoO2, which has a high energy density, is frequently utilized in consumer electronics. It is, nevertheless, somewhat costly and presents a safety issue because of thermal instability.
- ● Lithium Iron Phosphate (LiFePO4): LiFePO4's outstanding thermal stability and safety make it an excellent option for high-reliability applications like electric cars and power equipment. Its lower energy density is the price paid for its enhanced safety profile.
- ● Lithium Nickel Manganese Cobalt Oxide (NMC): NMC balances high energy density, good thermal stability, and reasonable cost, making it popular for electric vehicles and grid storage. The exact ratio of nickel, manganese, and cobalt can be adjusted to optimize specific performance characteristics.
- ● Lithium Manganese Oxide (LiMn2O4): LiMn2O4 provides good thermal stability and safety, with moderate energy density. It is often used in power tools and some electric vehicles.
3. Electrolyte
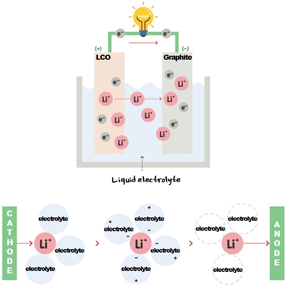
Figure 4
The electrolyte in a lithium-ion battery facilitates the transfer of lithium ions from the anode to the cathode. Usually, an organic solvent is used to dissolve a lithium salt. The most typical electrolyte mixture consists of:
- ● Lithium Hexafluorophosphate (LiPF6): This lithium salt is widely used due to its good electrochemical stability and high ionic conductivity.
- ● Natural Solvents: Common natural solvents are methyl methyl carbonate (EMC), dimethyl carbonate (DMC), ethylene carbonate (EC), and diethyl carbonate (DEC). These solvents were chosen because they provide a stable ion-transporting medium and have the ability to dissolve lithium salts.
The electrolyte must possess high ionic conductivity and stability over a wide temperature range while being compatible with the electrode materials. However, because of their flammability and leaking potential, liquid electrolytes present safety hazards. To address these concerns, researchers are exploring solid-state electrolytes, which offer improved safety and higher energy densities.
4. Separator

Figure 5
The separator is a crucial safety component in a lithium-ion battery. It is a microporous polymer layer that lets lithium ions flow through but blocks anode and cathode physical contact. Key characteristics of separators include:
- ● High Porosity: Ensures efficient ion transport while maintaining electrical insulation between the electrodes.
- ● Mechanical Strength: Provides structural integrity to withstand the stresses of battery operation and prevent short circuits.
- ● Thermal Stability: Maintains performance over a wide temperature range and can shut down ion flow in case of overheating, preventing thermal runaway.
Polypropylene (PP) and polyethylene (PE), two polyolefin polymers, are commonly utilized as separator materials. Advanced separators may incorporate ceramic coatings to enhance thermal stability and safety.
5. Current Collectors
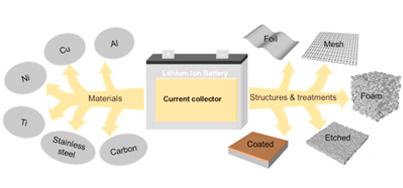
Figure 6
The movement of electrons from the electrodes to the external circuit is facilitated in a lithium-ion battery by current collectors. They are thin metal foils with high electrical conductivity and stability. The two primary current collectors are:
- ● Aluminum Foil: Used for the cathode due to its low density, high conductivity, and resistance to oxidation. Aluminum's light weight contributes to the overall energy density of the battery.
- ● Copper Foil: Used for the anode because of its excellent electrical conductivity and stability with lithium. Copper guarantees effective electron transfer throughout the charging and discharging procedures.
The current collectors must also have good adhesion to the electrode materials to ensure efficient electron transfer and mechanical stability during battery operation.
The anode, cathode, electrolyte, separator, and current collectors that make up the complex structure of lithium-ion batteries are carefully engineered to offer high energy density, extended cycle life, and safety. Every part is essential to the battery's overall function, and research is always being done to improve these parts even more. Understanding the detailed structure of lithium-ion batteries helps appreciate their complexity and the engineering challenges involved in their development and optimization.
Ⅲ. Working Principle of Lithium-ion Batteries
The primary mechanism by which lithium ions migrate from the anode to the cathode in lithium-ion batteries is electrochemical reaction. Electrical power is produced by the electrons flowing through an external circuit in tandem with the passage of ions through the electrolyte. The processes of charging and discharging involve several key steps and mechanisms.
1. Charging Process
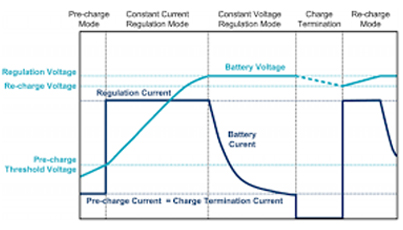
Figure 7
Lithium ions are driven from the cathode to the anode during the charging process by an external power source at a voltage higher than the battery's open circuit voltage. This process includes three main stages: deintercalation, migration, and intercalation.
Deintercalation
The first step in removing lithium ions from the cathode material is called deintercalation. In the case of a lithium cobalt oxide (LiCoO2) cathode, the process can be represented as follows:
Here, lithium ions (Li^+) leave the cathode material, resulting in the oxidation of cobalt from Co^3+ to Co^4+ and the release of electrons.
Migration
After being deintercalated from the cathode, lithium ions go through the electrolyte and in the direction of the anode.The electrolyte, which is typically a lithium salt (like LiPF6) mixed in an organic solvent, facilitates the movement of these ions. The positively charged lithium ions are drawn toward the negatively charged anode by the electric field created by the external charger, which is what drives the migration.
Intercalation
Upon reaching the anode, the lithium ions are intercalated into the anode material, which is usually graphite. The process is as follows:
Lithium ions insert themselves between the layers of graphite, and the accompanying electrons provided by the external circuit balance the charge. This intercalation process effectively stores energy in the battery.
Electron Flow
Flow of Electrons Through the external circuit, electrons move synchronously from the cathode to the anode. These electrons flow and complete the circuit as a result of the potential difference the charger generates, balancing the overall charge transfer that takes place during the charging process.
2. Procedure for Discharging
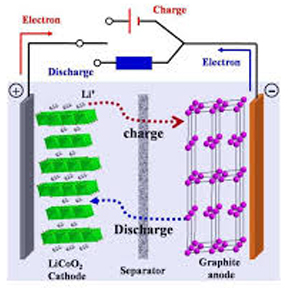
Figure 8
The battery's chemical energy is transformed back into electrical energy while discharge, enabling the linked device to function. The procedure, which includes deintercalation, migration, and intercalation, is basically the opposite of charging.
Deintercalation
During discharge, lithium ions are deintercalated from the anode material (graphite):
The electrons are discharged into the external circuit and the lithium ions exit the graphite layers.
Migration
Back through the electrolyte and toward the cathode go the deintercalated lithium ions. Due to the electrochemical potential difference between the anode and cathode, lithium ions are propelled forward.
Intercalation
At the cathode, the lithium ions are intercalated back into the cathode material:
When lithium ions enter the cathode, the original compound is restored. Through the external circuit, electrons go from the anode to the cathode, giving the linked device electrical energy.
Electron Flow
To power the attached device, electrons go from the anode to the cathode via the external circuit. The electric current that the device runs on is made up of this electron flux.
3. Electrochemical Reactions
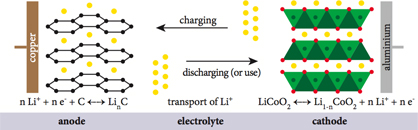
Figure 9
The electrochemical reactions occurring in a lithium-ion battery can be summarized as follows:
At the Anode (during discharge):
At the Cathode (during discharge):
The overall cell reaction of a lithium-ion battery that has a lithium cobalt oxide cathode and graphite anode is:
During charging, the reactions are reversed:
- ● At the anode:
- ● At the cathode:
4. Detailed Mechanisms and Kinetics
Ion Transport
The transport of lithium ions within the electrolyte involves both migration under an electric field and diffusion due to concentration gradients. The electrolyte's ionic conductivity and the mobility of lithium ions are crucial for the battery's performance.
Interphase Solid-Electrolyte (SEI)
On the anode surface, a solid-electrolyte interphase (SEI) layer develops during the first charging cycles. This layer affects the battery's longevity and performance. It stops the electrolyte from continuously breaking down, letting lithium ions flow through but keeping electrons out. The cycle life of the battery is extended by a stable SEI layer, which shields the anode material from additional electrolyte reaction.
Intercalation/Deintercalation Kinetics
Lithium ions are inserted into and removed from the electrode materials during the intercalation and deintercalation processes. The structural integrity of the electrode, the surface area of the electrode materials, and the diffusion coefficients of lithium ions within the electrodes all have an impact on these processes. Rapid intercalation/deintercalation kinetics are necessary for effective energy storage and high power density.
The reversible migration of lithium ions across the electrolyte between the anode and cathode, while electrons flow through an external circuit, is the fundamental mechanism of lithium-ion batteries. Understanding the detailed processes of charging and discharging, along with the associated electrochemical reactions, provides insight into how these batteries deliver power efficiently and reliably. The intricate mechanisms, including ion transport, SEI formation, and intercalation kinetics, play crucial roles in the overall performance and longevity of lithium-ion batteries. As research and development continue, advancements in these areas will further enhance battery technology, leading to more efficient, safer, and longer-lasting energy storage solutions.
Ⅳ. Packaging of Lithium-ion Batteries
Lithium-ion batteries are designed in various shapes and sizes to cater to specific applications, ensuring optimal performance, safety, and efficiency. These batteries' total performance is significantly influenced by the way they are packaged. This section provides an in-depth look at the different types of lithium-ion battery packaging, their benefits, challenges, and applications.
1. Cylindrical Cells

Figure 10
Cylindrical cells are among the most common and widely used formats for lithium-ion batteries. They are typically found in portable electronics, power tools, and electric vehicles. The two most popular cylindrical cell sizes are the 18650 and 21700 formats.
Advantages
- ● Mechanical Stability: The cylindrical shape offers high mechanical stability and structural integrity, making these cells robust and durable.
- ● Ease of Manufacture: Cylindrical cells are relatively simple to manufacture due to standardized processes and well-established production lines.
- ● Thermal management: Effective heat dissipation is made possible by the shape of cylindrical cells, which is essential for sustaining performance and averting overheating. By promoting uniform heat dispersion, the cylindrical shape lowers the possibility of thermal hotspots.
Applications
- ● Electronics that are portable: Found in gadgets like power banks, computers, and cameras.
- ● Electric Vehicles (EVs): Widely used in electric cars for their reliability and ease of integration into battery packs.
- ● Power Tools: Ideal for high-drain applications due to their robustness and energy density.
-
2. Prismatic Cells
Figure 11
Prismatic cells have a rectangular form factor, which makes them more space-efficient compared to cylindrical cells. They are frequently utilized in applications like consumer electronics and electric vehicles, where making the best use of available space is essential.
Advantages
- ● Space Efficiency: The rectangular shape allows for better utilization of space within a device or battery pack, making prismatic cells ideal for compact designs.
- ● Higher Capacity Design: Prismatic cells can be designed with higher capacities due to their larger form factor and optimized space usage.
- ● Thermal Management: While they are more space-efficient, prismatic cells can face challenges with thermal management and may require additional cooling mechanisms.
Challenges
- ● Swelling: Prismatic cells are more prone to swelling over time due to the buildup of internal pressure, which can affect performance and safety.
- ● Manufacturing Complexity: Prismmatic cells can be more expensive to produce and need a more complicated manufacturing procedure than cylindrical cells.
Applications
- ● Smartphones: Preferred for their high energy density and small form factor.
- ● Laptops: Preferred for their space efficiency and higher capacity.
- ● Electric Vehicles: Employed in EVs where space optimization is critical.
-
3. Pouch Cells

Figure 12
Pouch cells use a flexible, laminated aluminum foil casing, making them lightweight and adaptable to various shapes and sizes. This versatility is especially useful in applications where compact form factors and design freedom are crucial.
Advantages
- ● Lightweight and Compact: The pouch format eliminates the need for heavy metal casings, reducing the overall weight of the battery.
- ● High Energy Density: Pouch cells can achieve higher energy densities compared to other formats due to their efficient use of space.
- ● Flexible Design: The flexible casing allows for various shapes and sizes, enabling innovative product designs.
Challenges
- ● Mechanical Protection: Pouch cells require careful handling and robust packaging to prevent punctures and physical damage.
- ● Swelling: Like prismatic cells, pouch cells can swell due to gas buildup, requiring careful management of internal pressures.
Applications
- ● Tablets and Smartphones: Preferred for their thin profile and adaptability to different shapes.
- ● Electric vehicles: Used in EVs when space and weight are critical considerations.
- ● Wearable Devices: Ideal for applications requiring flexible and lightweight batteries.
-
4. Battery Modules and Packs

Figure 13
For applications demanding higher capacities and voltages, individual lithium-ion cells are assembled into battery modules and packs. This modular strategy enables customization and scalability to satisfy particular power and energy needs.
Construction
- ● Connections in Series and Parallel: Series connections enhance voltage, whereas parallel connections increase capacity. Because of its adaptability, designers can customize the battery pack to meet the unique requirements of the application.
- ● Integration of BMS: To ensure safe and effective operation, battery packs come equipped with a Battery Management System (BMS) that tracks and regulates each cell's performance.
Advantages
- ● Scalability: Battery modules and packs can be scaled up or down to match the power and energy requirements of different applications.
- ● Customization: Cell arrangements can be set up by designers to maximize efficiency for certain use cases.
Applications
- ● Electric Vehicles: Battery packs power EVs, providing the necessary energy and power density for extended driving ranges.
- ● Grid Storage: Used to maintain grid stability and store renewable energy in energy storage systems.
- ● Industrial Applications: Employed in backup power systems and large-scale industrial machinery.
-
5. Battery Management System (BMS)

Figure 14
The BMS is an integral component of a lithium-ion battery pack, responsible for ensuring safe and efficient operation by monitoring and managing the performance of individual cells within the pack.
Key Functions
- ● Cell balancing: Prevents individual cells from being overcharged or overdischarged by ensuring that all of the cells in the pack maintain the same level of charge.
- ● Protection: Detects and responds to conditions such as overvoltage, undervoltage, overcurrent, and overtemperature, protecting the battery pack from damage.
- ● State Monitoring: Provides information on the state of charge (SOC), state of health (SOH), and remaining capacity of the battery pack, enabling predictive maintenance and efficient use.
-
6. Thermal Management
Figure 15
Effective thermal management is critical for maintaining the performance and safety of lithium-ion batteries. Overheating, which can result in deterioration, decreased performance, and safety risks, is avoided by proper thermal regulation.
Strategies
- ● Air cooling: To release heat, fans or natural convection are used. Although this approach is easy to use and reasonably priced, it might not be enough for high-power applications.
- ● Liquid Cooling: Circulates coolant through channels or plates within the battery pack to remove heat. This method offers more effective thermal regulation, particularly in high-power and high-density applications.
- ● Phase Change Materials (PCMs): To maintain stable temperatures during phase changes, materials that absorb and release heat are used. The need for active cooling systems may be reduced by PCMs' passive thermal management.
Applications
- ● Electric Vehicles: Advanced thermal management systems are essential to maintain battery performance and safety in EVs, where large amounts of energy are stored and discharged.
- ● Consumer Electronics: Efficient thermal management extends battery life and ensures safe operation in devices such as laptops and smartphones.
- ● Energy Storage Systems: Effective cooling systems are necessary to handle the large energy throughput in grid storage applications.
The packaging of lithium-ion batteries is a critical aspect of their design, directly impacting their performance, safety, and applicability. Different usage can benefit from the distinct advantages and disadvantages of prism, pouch, and cylindrical cells. Battery modules and packs, equipped with sophisticated BMS and thermal management systems, enable the scalable and efficient use of lithium-ion technology in various industries. As the demand for high-performance, reliable, and safe energy storage solutions continues to grow, advancements in battery packaging will play a pivotal role in meeting these needs.
Ⅴ. Primary apparatus for producing lithium-ion batteries
1. Mixing Equipment:

Figure 16
Used to mix the active materials, conductive additives, and binders to create a slurry. This includes mixers and homogenizers.
2. Coating Machine:

Figure 17
Coats the slurry onto the current collector foil (cathode and anode) to create electrode sheets.
3. Drying Oven:

Figure 18
Dries the coated electrode sheets to remove solvents and achieve the desired electrode properties.
4. Calendering Machine:

Figure 19
Presses the electrode sheets to the desired thickness and density, improving the contact between active materials and the current collector.
5. Slitting Machine:

Figure 20
Cuts the electrode sheets into narrow strips that are suitable for the cell design.
6. Electrode Stacking/Winding Machine:

Figure 21
Assembles the electrodes into a stack (for prismatic cells) or winds them into a roll (for cylindrical and pouch cells).
7. Tab Welding Machine:

Figure 22
Welds the current collectors to the cell tabs, ensuring good electrical connectivity.
8. Cell Assembly Machine:

Figure 23
Encloses the electrodes and electrolyte into the cell casing, which can be cylindrical, prismatic, or pouch types.
9. Electrolyte Filling Machine:

Figure 24
Fills the cells with electrolyte, which is critical for the ion transport within the battery.
10. Sealing Machine:

Figure 25
Seals the cells to prevent electrolyte leakage and to maintain the internal pressure.
11. Testing Equipment:

Figure 26
Includes equipment for electrical testing (capacity, voltage, resistance), safety testing (short circuit, overcharge, crush), and quality control (X-ray inspection, laser scanning).
Ⅵ. Advantages and Challenges of Lithium-ion Batteries
Energy storage has been transformed by lithium-ion batteries in a number of industries, including renewable energy systems, electric cars, and portable devices. Although they are popular and have numerous benefits, they also have some significant drawbacks. We shall go into more detail about the benefits and drawbacks of lithium-ion batteries here.
1. Advantages
Increased Energy Density
A Higher Density of Energy The high energy density of lithium-ion batteries is one of its primary benefits. This feature is essential for applications that need a lot of power in a small package, like:
- ● Portable Electronics: Devices like smartphones, laptops, and tablets rely on lithium-ion batteries to provide long-lasting power while keeping the overall weight and size manageable.
- ● Electric Vehicles (EVs): Electric vehicles with high energy density have greater driving ranges than vehicles with internal combustion engines, which increases their competitiveness.
Generally speaking, lithium-ion batteries have an energy density of 150–250 Wh/kg, which is a lot more than other rechargeable battery technologies like nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries.
Long Cycle Life
Long-term use of lithium-ion batteries is financially feasible due to their ability to withstand hundreds to thousands of charge-discharge cycles with little loss of capacity. Among the main causes of their lengthy cycle life are:
- ● Stable Electrochemical Reactions: The intercalation and deintercalation processes of lithium ions in the electrodes are highly reversible, leading to less degradation over time.
- ● Advanced Battery Management Systems (BMS): Modern lithium-ion battery packs are equipped with BMS that monitor and manage the charging process, preventing conditions that could lead to rapid degradation.
For example, electric vehicle batteries are often designed to last for more than 1,000 cycles or over ten years of regular use, which aligns with the vehicle's lifespan.
Low Self-Discharge
Minimal Self-Discharge Lithium-ion batteries have a far lower self-discharge rate than other rechargeable batteries. When not in use, they usually only lose 1% to 2% of their charge monthly. This quality is especially advantageous for:
- ● Backup Power Systems: Batteries with low self-discharge are dependable for emergency power since they hold their charge for longer.
- ● Consumer Electronics: Devices can remain charged for longer periods, even when not used regularly, providing convenience and reliability.
Lightweight
Lithium and aluminum, two lightweight components utilized in lithium-ion batteries, help to reduce the weight of the battery overall. For situations where weight is a major factor, this benefit is essential:
- ● Portable Electronics: Lightweight batteries enhance the portability of devices, making them easier to carry and use.
- ● Aerospace and Electric Vehicles: In these industries, reducing weight can lead to better performance and efficiency, such as increased flight range for drones or longer driving range for electric vehicles.
-
2. Challenges
Safety Concerns
Lithium-ion batteries have benefits, but if used improperly, they can be dangerous. Important safety issues consist of:
- ● Thermal Runaway: This problem, in which the battery produces excessive heat uncontrollably, can be brought on by overcharging, overdischarging, or exposure to extreme temperatures. Explosions or fires could result from this.
- ● Short Circuits: Physical damage or manufacturing defects can lead to internal short circuits, triggering thermal runaway.
- ● Chemical Stability: The highly reactive nature of lithium and the organic solvents used in the electrolyte can pose fire hazards if the battery is damaged or improperly handled.
To mitigate these risks, manufacturers incorporate several safety features, such as:
- ● Internal Circuit Protection: Features like pressure-sensitive vents and thermal fuses.
- ● Advanced battery management systems (BMS) avoid overcharging and overheating by monitoring and controlling the charge and discharge processes.
Cost
The production of lithium-ion batteries involves costly materials and complex manufacturing processes, contributing to their higher price compared to other battery types. Key cost factors include:
- ● Raw Materials: Materials like lithium, cobalt, and nickel are expensive and have volatile market prices.
- ● Manufacturing Technology: The processes required to produce high-quality lithium-ion cells are sophisticated and capital-intensive.
Costs are being lowered, nevertheless, by technology breakthroughs and economies of scale. For example, over the past ten years, the price of lithium-ion batteries for electric cars has decreased dramatically, bringing EVs closer to the mainstream.
Reusing and Throwing Out
It's important to dispose of and recycle lithium-ion batteries properly to lessen their harmful effects on the environment.. Obstacles in this field consist of:
- ● Complicated Recycling Procedures: The recycling procedure entails the costly and technically difficult separation and recovery of valuable materials such as nickel, cobalt, and lithium.
- ● Environmental Hazards: Improper disposal of hazardous items can cause leaks into the environment, posing a risk to human health and ecosystems.
Technologies and procedures for recycling are being improved. For instance, efforts are being made to create more economical and effective recycling techniques in order to improve the recovery of valuable materials and lessen the impact on the environment.
Resource Availability
Concerns over the availability and moral sourcing of essential materials like lithium, cobalt, and nickel have been brought up by the growing demand for lithium-ion batteries. Important concerns consist of:
- ● Constraints on the Supply Chain: Market demand, mining laws, and geopolitical conditions can all have an impact on the availability of these resources.
- ● Ethical Sourcing: Demands for more sustainable and ethical sourcing methods have arisen from worries about abuses of human rights and environmental damage in cobalt mining locations.
To address these challenges, research is focused on:
- ● Alternative Materials: Developing batteries with reduced reliance on scarce or ethically problematic materials.
- ● Sustainable Practices: Improving mining practices and establishing recycling programs to ensure a steady supply of raw materials.
High energy density, extended cycle life, less self-discharge, and lightweight design are just a few of the benefits that make lithium-ion batteries perfect for a variety of uses. They do, however, also have to deal with a number of formidable obstacles, including resource shortages, safety concerns, high costs, and recycling and disposal issues. Lithium-ion battery technology will need to address these issues through technological developments, enhanced production techniques, and sustainable business practices in order to continue growing and being adopted.
Ⅶ. Future Developments in Lithium-ion Batteries
The future of lithium-ion batteries is centered on continuous improvement and innovation, addressing current limitations while enhancing their performance and sustainability. Key areas of research and development are crucial in driving the next generation of lithium-ion batteries.
1. Solid-State Batteries
By substituting a solid electrolyte for the liquid electrolyte in conventional lithium-ion batteries, solid-state batteries offer a substantial improvement over their counterparts. This change brings several potential benefits:
Improved Safety
- ● Non-Flammable: Compared to liquid electrolytes, solid electrolytes often do not catch fire or burst into explosions.
- ● Stability: One of the main safety concerns with lithium-ion batteries is thermal runaway, which can be avoided by using solid electrolytes, which are more stable at higher temperatures.
Enhanced Energy Density
- ● Thin Layers: Solid-state batteries can use very thin layers of solid electrolytes, allowing for a more compact design and higher energy density.
- ● Dendrite Prevention: The solid electrolyte can prevent the formation of lithium dendrites, which can short-circuit the battery, thereby enabling the use of high-capacity lithium metal anodes.
Longer Cycle Life
- ● Reduced Degradation: Solid-state batteries tend to have lower rates of degradation due to the stability of the solid electrolyte, leading to a longer cycle life.
Challenges
- ● Manufacturing Complexity: Developing cost-effective and scalable manufacturing processes for solid-state batteries remains a challenge.
- ● Material Compatibility: The solid electrolyte and electrode materials must be suitable for efficient ion transport and overall battery performance.
-
2. Superior Anode Materials
Creating more sophisticated anode materials is a major area of interest for improving the performance and energy density of lithium-ion batteries.
Silicon Anodes
- ● High Capacity: Silicon can theoretically store ten times more lithium ions than graphite, significantly increasing the battery's capacity.
- ● Volume Expansion: When silicon anodes are charged and discharged, they experience significant volume fluctuations that may cause mechanical deterioration. Researchers are exploring nano-engineered structures and silicon composites to mitigate this issue.
Lithium Titanium Oxide (LTO) Anodes
- ● Safety and Stability: LTO anodes offer excellent safety, thermal stability, and long cycle life. They are less prone to forming a solid-electrolyte interphase (SEI) layer, which can improve the overall longevity of the battery.
- ● Lower Energy Density: LTO anodes are safer than silicon or graphite anodes, but they are still suited for applications where long life and safety are more important than energy density.
-
3. Cobalt-Free Cathodes
Reducing or eliminating cobalt in cathode materials addresses both cost and ethical sourcing issues. Research is focused on developing alternative materials that maintain or improve battery performance.
Lithium Iron Phosphate (LiFePO4)
- ● Safety and Stability: LiFePO4 is renowned for having exceptional safety and heat stability. It is less likely to experience thermal runaway or overheat.
- ● Lower Energy Density: It has a lower energy density compared to cobalt-based cathodes, but improvements in cell design are helping to bridge this gap.
Lithium Nickel Manganese Oxide (LNMO)
- ● High Voltage: LNMO operates at a higher voltage than traditional cathode materials, which can increase the overall energy density of the battery.
- ● Cobalt-Free: Completely eliminates cobalt, reducing costs and addressing ethical concerns.
-
4. Fast Charging
Enhancing the charging speed of lithium-ion batteries without compromising safety or cycle life is critical for consumer convenience and the adoption of electric vehicles.
Improved Electrolytes
- ● High Ionic Conductivity: Developing electrolytes with higher ionic conductivity can reduce the internal resistance of the battery, allowing for faster charging.
- ● Stability: Ensuring the stability of electrolytes at higher voltages and temperatures is essential to maintain safety during fast charging.
Advanced Charging Protocols
- ● Smart Charging Algorithms: Implementing advanced algorithms that dynamically adjust the charging rate based on the battery's state can optimize the charging process, reducing stress on the battery and extending its life.
- ● Pulse Charging: Using pulse charging techniques can help minimize heat generation and improve the overall efficiency of the charging process.
-
5. Technologies for Recycling
Improving lithium-ion battery recycling techniques is essential to reclaiming valuable materials and lessening the negative effects on the environment.
Efficient Material Recovery
- ● Hydrometallurgical Processes: These processes involve using aqueous solutions to recover metals from spent batteries, offering high recovery rates and lower environmental impact compared to traditional pyrometallurgical methods.
- ● Direct Recycling: This approach aims to recover and reuse battery components (such as cathode and anode materials) without breaking them down into elemental forms, preserving their structure and reducing the need for extensive reprocessing.
Environmental and Economic Benefits
- ● Reducing Waste: Effective recycling reduces the volume of hazardous waste generated by spent batteries, minimizing environmental contamination.
- ● Resource conservation: Recycling helps to recover vital resources like nickel, cobalt, and lithium, which lessens the demand for new mining and eases supply chain constraints.
Policy and Regulation
- ● Extended Producer Responsibility (EPR): Manufacturers can be encouraged to design batteries with recycling in mind and assume accountability for their end-of-life disposal by enacting EPR laws.
- ● Standardization: Developing standardized processes and regulations for battery recycling can improve efficiency and ensure consistent recovery of valuable materials.
The future of lithium-ion batteries lies in continuous innovation and addressing current limitations. Solid-state batteries, advanced anode materials, cobalt-free cathodes, fast charging technologies, and improved recycling methods represent key areas of research and development. By improving battery performance, safety, and sustainability, these developments hope to open the door for a wider use of lithium-ion technology across a range of industries. As these technologies mature, they will play a pivotal role in powering the next generation of electronic devices, electric vehicles, and renewable energy systems, contributing to a more sustainable and energy-efficient future.
Ⅶ. Conclusion
Because lithium-ion batteries combine a high energy density, long cycle life, and durability, they have completely changed the way we power our gadgets and cars. Comprehending their composition, mode of operation, and packaging is essential to realizing their advantages and overcoming their drawbacks. As technology advances, lithium-ion batteries will continue to dominate the market for energy storage solutions, encouraging sustainability and creativity across a wide range of industries.
Common Queries Answered
1. What benefits do lithium-ion batteries have over other battery types?
Lithium-ion batteries' high energy density, long cycle life, minimal self-discharge, lightweight construction, and excellent efficiency make them ideal for portable devices, electric vehicles, and renewable energy storage.
2. How operate batteries made of lithium-ion?
During charging and discharging, lithium ions travel via an electrolyte from the anode to the cathode in lithium-ion batteries. Devices are powered by the electric current produced by this ion movement.
3. What constitutes a lithium-ion battery's principal parts?
The anode (usually graphite), cathode (generally lithium metal oxides), electrolyte (a lithium salt in an organic solvent), separator, and current collectors (a copper anode and an aluminum cathode) are the essential parts of a lithium-ion battery.
4. What is the average lifespan of lithium-ion batteries?
Lithium-ion batteries typically last between 500 to 1,500 charge cycles, which can equate to several years of use depending on the application and usage patterns. Electric vehicle batteries, for example, are often designed to last 8-10 years.
5. Which safety issues surround lithium-ion batteries?
Lithium-ion battery safety issues include the potential for thermal runaway, fires, and explosions brought on by physical damage, overcharging, or overheating. To reduce these dangers, effective battery management systems (BMS) and safety measures are essential.
6. How is the recycling process for lithium-ion batteries carried out?
The recycling process for lithium-ion batteries involves collecting and sorting batteries, extracting valuable materials like lithium, cobalt, and nickel using hydrometallurgical or pyrometallurgical methods, and reusing or disposing of remaining materials safely.
7. What recent developments have occurred in the field of lithium-ion batteries?
Recent advancements include solid-state batteries, silicon anodes, cobalt-free cathodes, fast-charging technologies, and improved recycling methods. These innovations aim to enhance energy density, safety, and sustainability.
8. Why are lithium-ion batteries used in electric vehicles?
Because of its high energy density, which enables longer driving ranges, quick charging times, and extended cycle lives, lithium-ion batteries are utilized in electric vehicles (EVs) and meet the rigorous specifications of these vehicles.
9. What are the common applications for lithium-ion batteries?
Because they are dependable, efficient, and small in size, they are frequently used in power tools, medical equipment, electric cars, cycles, portable electronics (such as laptops and smartphones), and renewable energy storage systems.
10. How can I make my lithium-ion battery last longer?
To extend the life of a lithium-ion battery, avoid extreme temperatures, prevent full discharges and overcharges, use appropriate chargers, store batteries partially charged if not in use for long periods, and follow manufacturer guidelines for usage and maintenance.
Related Information
-
-
Phone
+86 135 3401 3447 -
Whatsapp





