Exploring the reference electrode: an indispensable magical element in electrochemistry
2024/5/13 14:37:04
Views:
The reference electrode is an indispensable component in electrochemical research and practical applications, playing a key role in measuring and controlling electrical potential. As a basic unit of an electrochemical cell, the function of the reference electrode is to provide a constant and known potential reference in order to accurately determine the relative potential changes on the working electrode (such as the site where redox reactions occur). The working theory, key traits, and particular uses of reference electrodes in many domains will all be covered in this article.
The reference electrode usually consists of two parts: one is a substance with a stable standard potential, such as a saturated calomel electrode (SCE), a hydrogen electrode, etc.; the other is a conductive medium, such as an electrolyte solution. When the reference electrode is inserted into the system being measured, its electrode surface will form a stable charge exchange interface, thereby maintaining a fixed potential value. This fixed potential value provides a reference point for the potentials of other electrodes, allowing the electrochemical reaction potential on the working electrode to be accurately determined by comparing the voltage difference between the reference electrode and the working electrode.
Reference electrodes play a vital role in electrochemical experiments or analyses. It is an electrode used to provide a reference point of known electrical potential from which electrical potential measurements can be performed. The reference electrode is usually made of a stable material with a known potential and is connected to the electrode under test to determine the potential difference between the electrodes under test. Specifically, the main functions of the reference electrode are as follows:
1. Provide a known potential reference point: The reference electrode has a fixed potential and can be used repeatedly to provide a known potential reference point for measuring the potential of other electrodes.
2. Determine the concentration of substances in the electrolyte: The reference electrode can be used to determine the concentration of certain substances in the electrolyte or perform quantitative analysis of the solution, such as acidity determination, metal ion determination, and redox potential determination.
3. Control the potential difference between electrodes: In many electrochemical reaction processes, the reference electrode is also used to control the potential difference between electrodes to ensure the accuracy and stability of the experiment.
In order to ensure the accuracy of the reference electrode, it needs to meet some special conditions, such as having a stable and repeatable potential, being able to maintain high stability in solution, and not being oxidized or reduced by the electrode or reactant under test. Commonly used reference electrodes include slightly soluble salt electrodes, etc. Although the hydrogen electrode is an ideal reference electrode, it is not commonly used in practical applications because it is not easy to implement.
Important Characteristics of Reference Electrodes
1. Stability: An ideal reference electrode should have a high degree of potential stability, that is, under certain conditions, the change in electrode potential is extremely small or negligible, so as to ensure the reliability of the measurement results.
2. Reproducibility: A good reference electrode should be able to be used repeatedly under different experimental conditions and produce consistent potential readings, which is crucial for scientific research and industrial production process monitoring.
3. Fast response time: A fast-responding reference electrode can reflect the dynamic changes of the electrochemical system in real time, which is particularly important when performing transient potential analysis.
4. Compatibility: The reference electrode needs to be compatible with the electrolyte of the system being tested, and will not change its own electrode potential or affect the electrochemical properties of the system due to interaction.
Application examples of reference electrodes
1. Electrochemical sensors and detection technology: In the fields of water quality monitoring, environmental pollutant detection, and biological sensing, reference electrodes are often used to construct a three-electrode system (working electrode, auxiliary electrode, and reference electrode). The potential of the redox reaction is monitored in real time to achieve accurate quantitative analysis of the concentration of the target substance.
2. Corrosion science and protection: In the anti-corrosion research of metal materials, the reference electrode is used to measure the corrosion potential of the metal surface, evaluate its corrosion tendency and corrosion rate, and help formulate effective anti-corrosion measures.
3. Energy field: In the research and optimization process of fuel cells and secondary batteries (such as lithium-ion batteries), the reference electrode is used to evaluate the performance of electrode materials and electrochemical reaction kinetic parameters to guide the design and improvement of new batteries.
4. Biomedical Engineering: in Neuroscience, Physiology In research, reference electrodes are often used to be implanted in the body to monitor nerve signals or changes in ion concentration inside/outside cells, such as playing an important role in electrocardiogram (ECG) and electroencephalogram (EEG) recording.
5. Electrolysis and electrodeposition processes: During electrochemical processing such as electroplating and electrolytic refining, the reference electrode is used to monitor and control the potential distribution in the electrolytic cell to ensure precise control of process conditions.
In conclusion, the electrochemical system relies heavily on the reference electrode. It is the basis for potential measurement and control, and has a profound impact on many fields such as scientific research and exploration, industrial production and even medical health. With the development of science and technology, the design and manufacturing of reference electrodes are also constantly improving to meet the needs of increasingly complex and diverse electrochemical application scenarios.
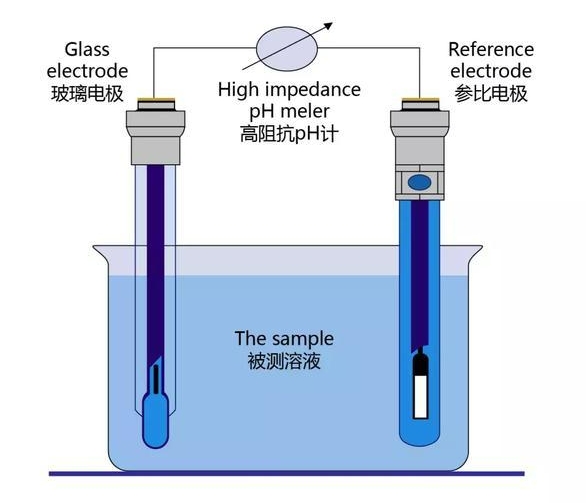
The reference electrode usually consists of two parts: one is a substance with a stable standard potential, such as a saturated calomel electrode (SCE), a hydrogen electrode, etc.; the other is a conductive medium, such as an electrolyte solution. When the reference electrode is inserted into the system being measured, its electrode surface will form a stable charge exchange interface, thereby maintaining a fixed potential value. This fixed potential value provides a reference point for the potentials of other electrodes, allowing the electrochemical reaction potential on the working electrode to be accurately determined by comparing the voltage difference between the reference electrode and the working electrode.
Reference electrodes play a vital role in electrochemical experiments or analyses. It is an electrode used to provide a reference point of known electrical potential from which electrical potential measurements can be performed. The reference electrode is usually made of a stable material with a known potential and is connected to the electrode under test to determine the potential difference between the electrodes under test. Specifically, the main functions of the reference electrode are as follows:
1. Provide a known potential reference point: The reference electrode has a fixed potential and can be used repeatedly to provide a known potential reference point for measuring the potential of other electrodes.
2. Determine the concentration of substances in the electrolyte: The reference electrode can be used to determine the concentration of certain substances in the electrolyte or perform quantitative analysis of the solution, such as acidity determination, metal ion determination, and redox potential determination.
3. Control the potential difference between electrodes: In many electrochemical reaction processes, the reference electrode is also used to control the potential difference between electrodes to ensure the accuracy and stability of the experiment.
In order to ensure the accuracy of the reference electrode, it needs to meet some special conditions, such as having a stable and repeatable potential, being able to maintain high stability in solution, and not being oxidized or reduced by the electrode or reactant under test. Commonly used reference electrodes include slightly soluble salt electrodes, etc. Although the hydrogen electrode is an ideal reference electrode, it is not commonly used in practical applications because it is not easy to implement.

Important Characteristics of Reference Electrodes
1. Stability: An ideal reference electrode should have a high degree of potential stability, that is, under certain conditions, the change in electrode potential is extremely small or negligible, so as to ensure the reliability of the measurement results.
2. Reproducibility: A good reference electrode should be able to be used repeatedly under different experimental conditions and produce consistent potential readings, which is crucial for scientific research and industrial production process monitoring.
3. Fast response time: A fast-responding reference electrode can reflect the dynamic changes of the electrochemical system in real time, which is particularly important when performing transient potential analysis.
4. Compatibility: The reference electrode needs to be compatible with the electrolyte of the system being tested, and will not change its own electrode potential or affect the electrochemical properties of the system due to interaction.
Application examples of reference electrodes
1. Electrochemical sensors and detection technology: In the fields of water quality monitoring, environmental pollutant detection, and biological sensing, reference electrodes are often used to construct a three-electrode system (working electrode, auxiliary electrode, and reference electrode). The potential of the redox reaction is monitored in real time to achieve accurate quantitative analysis of the concentration of the target substance.
2. Corrosion science and protection: In the anti-corrosion research of metal materials, the reference electrode is used to measure the corrosion potential of the metal surface, evaluate its corrosion tendency and corrosion rate, and help formulate effective anti-corrosion measures.
3. Energy field: In the research and optimization process of fuel cells and secondary batteries (such as lithium-ion batteries), the reference electrode is used to evaluate the performance of electrode materials and electrochemical reaction kinetic parameters to guide the design and improvement of new batteries.
4. Biomedical Engineering: in Neuroscience, Physiology In research, reference electrodes are often used to be implanted in the body to monitor nerve signals or changes in ion concentration inside/outside cells, such as playing an important role in electrocardiogram (ECG) and electroencephalogram (EEG) recording.
5. Electrolysis and electrodeposition processes: During electrochemical processing such as electroplating and electrolytic refining, the reference electrode is used to monitor and control the potential distribution in the electrolytic cell to ensure precise control of process conditions.
In conclusion, the electrochemical system relies heavily on the reference electrode. It is the basis for potential measurement and control, and has a profound impact on many fields such as scientific research and exploration, industrial production and even medical health. With the development of science and technology, the design and manufacturing of reference electrodes are also constantly improving to meet the needs of increasingly complex and diverse electrochemical application scenarios.
Related Information
-
-
Phone
+86 135 3401 3447 -
Whatsapp





